Site Selection & Harmonization
Defining the perfect fit-for-purpose site profile and harmonizing all the relevant procedures within is an essential part of a successful clinical trial.
Global Study Presence
20 years of global clinical trials experience taught us the special importance of standardizing cultural nuances and practices.
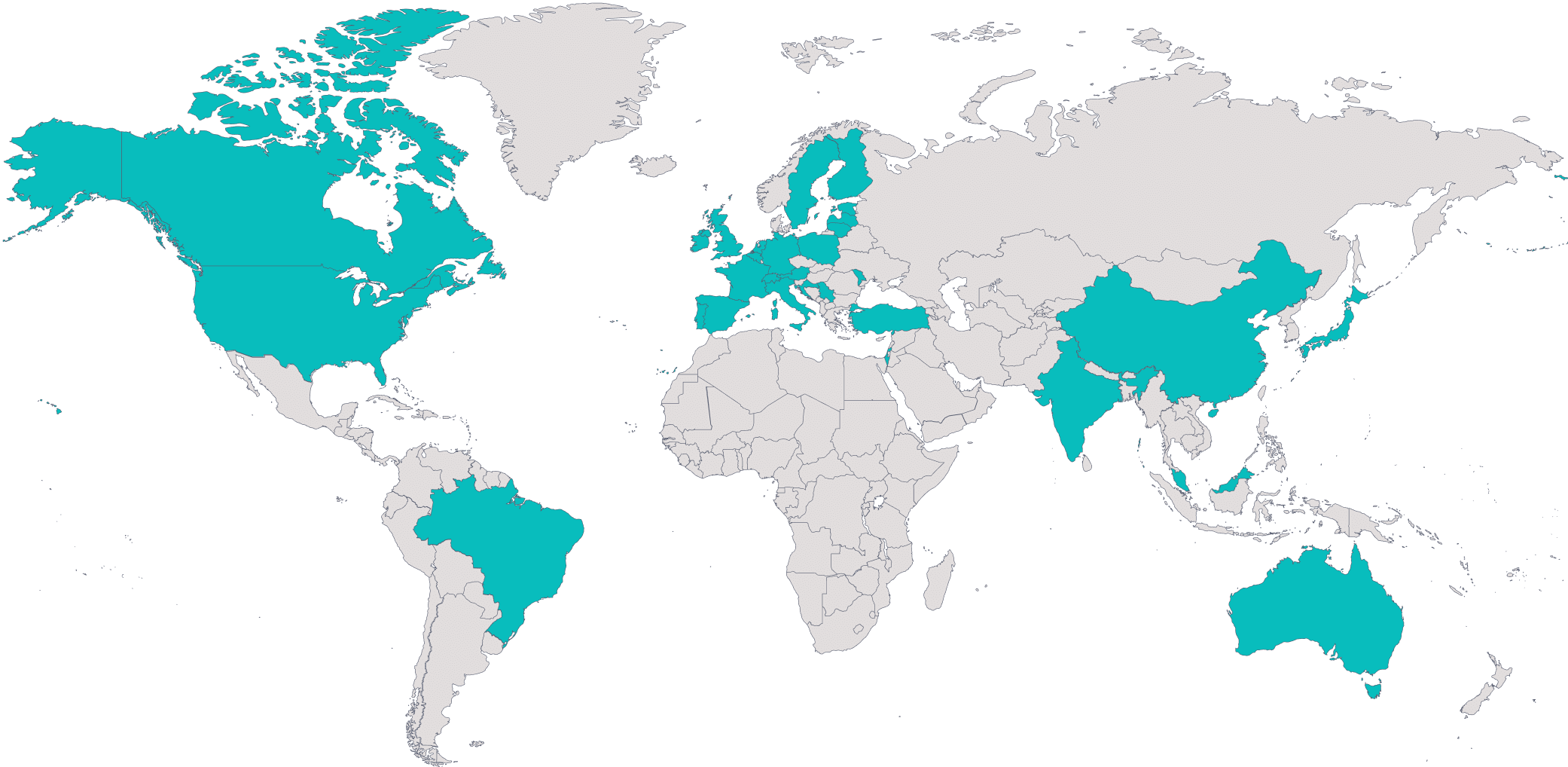
170+ US sites, 100+ EU sites and 50+ Asia/Pacific sites
Global PresenceHead office in Vienna (Austria), additional offices in Colmar (France) and New Jersey (US)
Site Harmonization
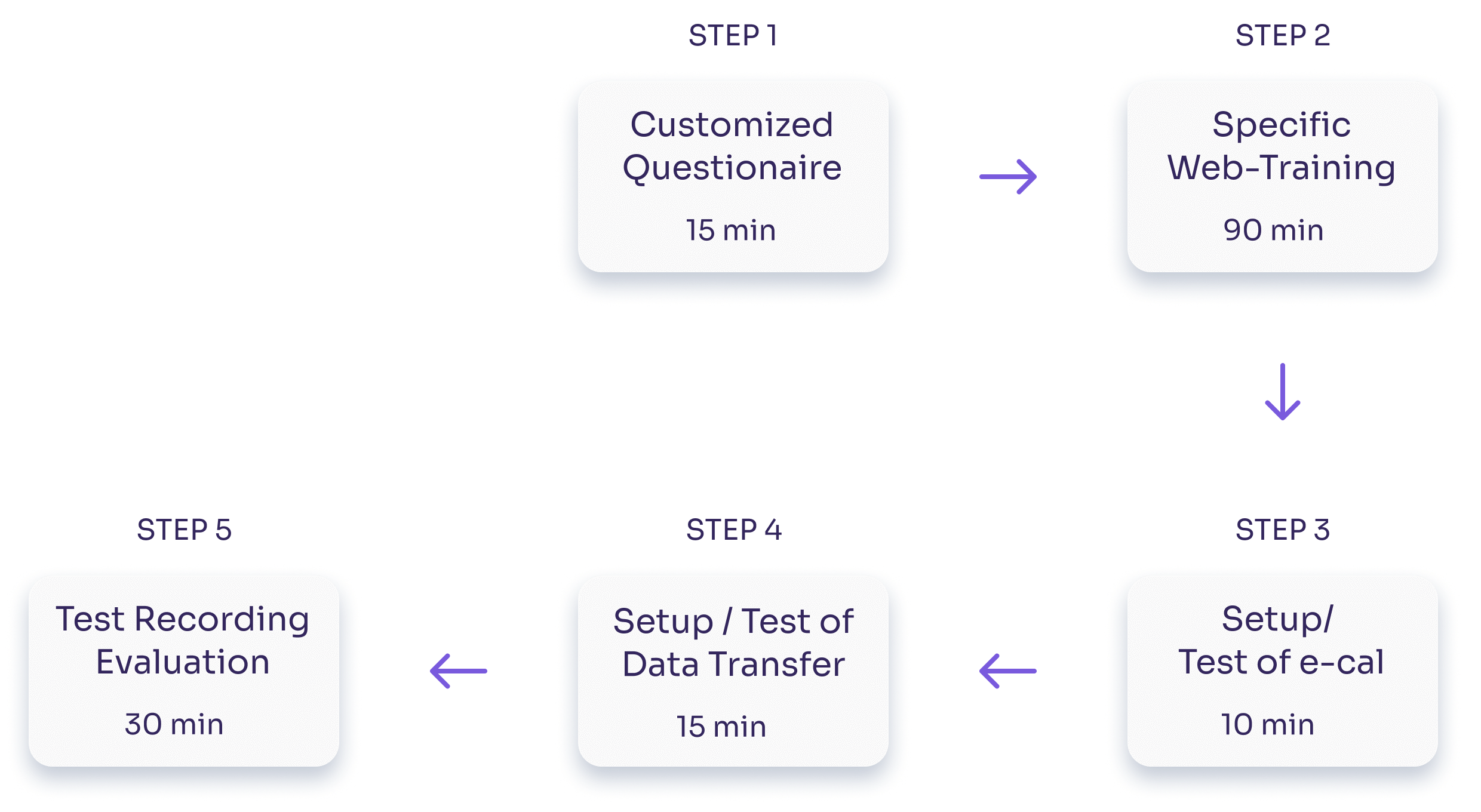
Our harmonization processes ensure complete compliance, regarding data format, protocol, study-specific procedures, recording devices, and signal quality.
Feasibility Test
- Assesses equipment and staff expertise. Resolves all potential.
- site-related issues such as correct device settings and data upload.
Site Instruction Manual
- Always protocol-specific, complete, and ensuring maximum harmonization across all sites of a multi-centre study.
Training Procedures
- Completely in parallel to all other start-up processes.
- Time-efficient and adapted to site expertise, but equally comprehensive and standardized.
Site Certification
Certification within a day!
With TSG, quality training does not hinder site readiness. We manage training so it is never a time- or rate-limiting factor.
CRO & Site Network
Interested in joining forces?
Benefit from our extensive expertise connecting CNS clinical trial operations with top of the line signal quality control and AI-powered central scoring /biomarker analysis.
Request Info