Trial Optimization
Streamlined clinical trial setup and operations right from biomarker consultation and protocol feasibility assessment to effective site management and device selection are imperative to accomplish optimal trial timelines and outcomes.
Study Design Consultation
We emphasize a goal-oriented, adaptive and partnering design consultation process because we are convinced that deeply understanding your research goals is the foundation to fully align our services to your CNS trial needs.
- site training (adapted to staff experience and study protocol)
- review & certification of test recordings
- detailed research of state-of-the-art device technologies
- critical review of applicability, feasibility, tolerability, and regulatory implications
- biomarker-specific power analysis and sample size estimates
- pro-active mitigation of confounders (e.g., placebo and first-night effect, baseline activity, psychometric measures)
End-to-End Study Partner
With our deep understanding of the key aspects guiding decision making processes during drug development, we support our partners at all stages of a CNS clinical trial.
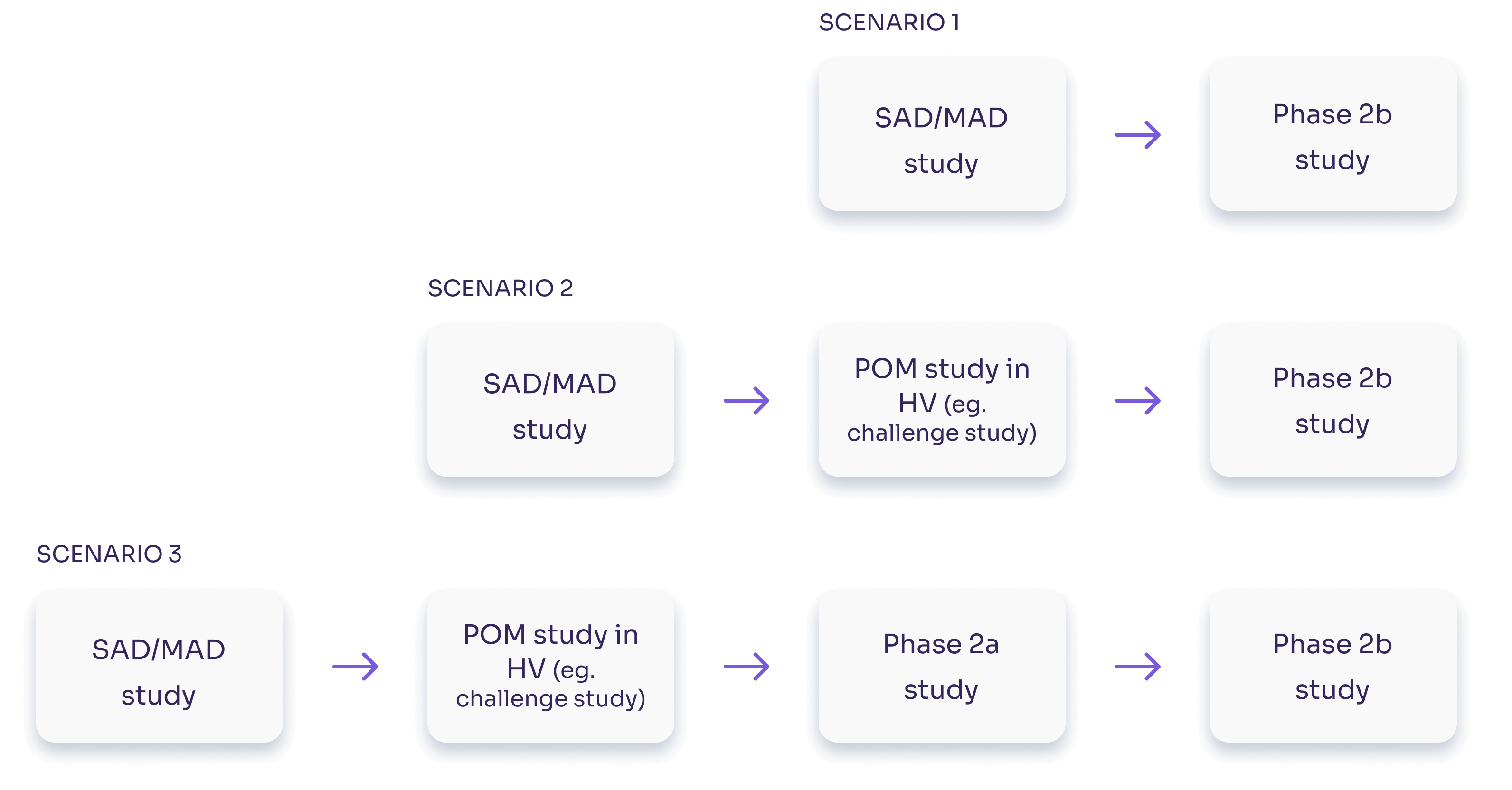
Tractability is critical. How well can your compound’s effects be tracked, across species?
Viability. We consult on the best fit-for-purpose biomarker profile based on disease model and disease state modulation.
Safety and Target Engagement. We implement endpoints with the highest signal quality possible.
Efficacy and Dosage. We help to demonstrate clinical relevance and to define effective dose ranges.
Up-scaling. With our worldwide study presence and extensive operational expertise we excel at supporting global multi-center and virtualized trials.
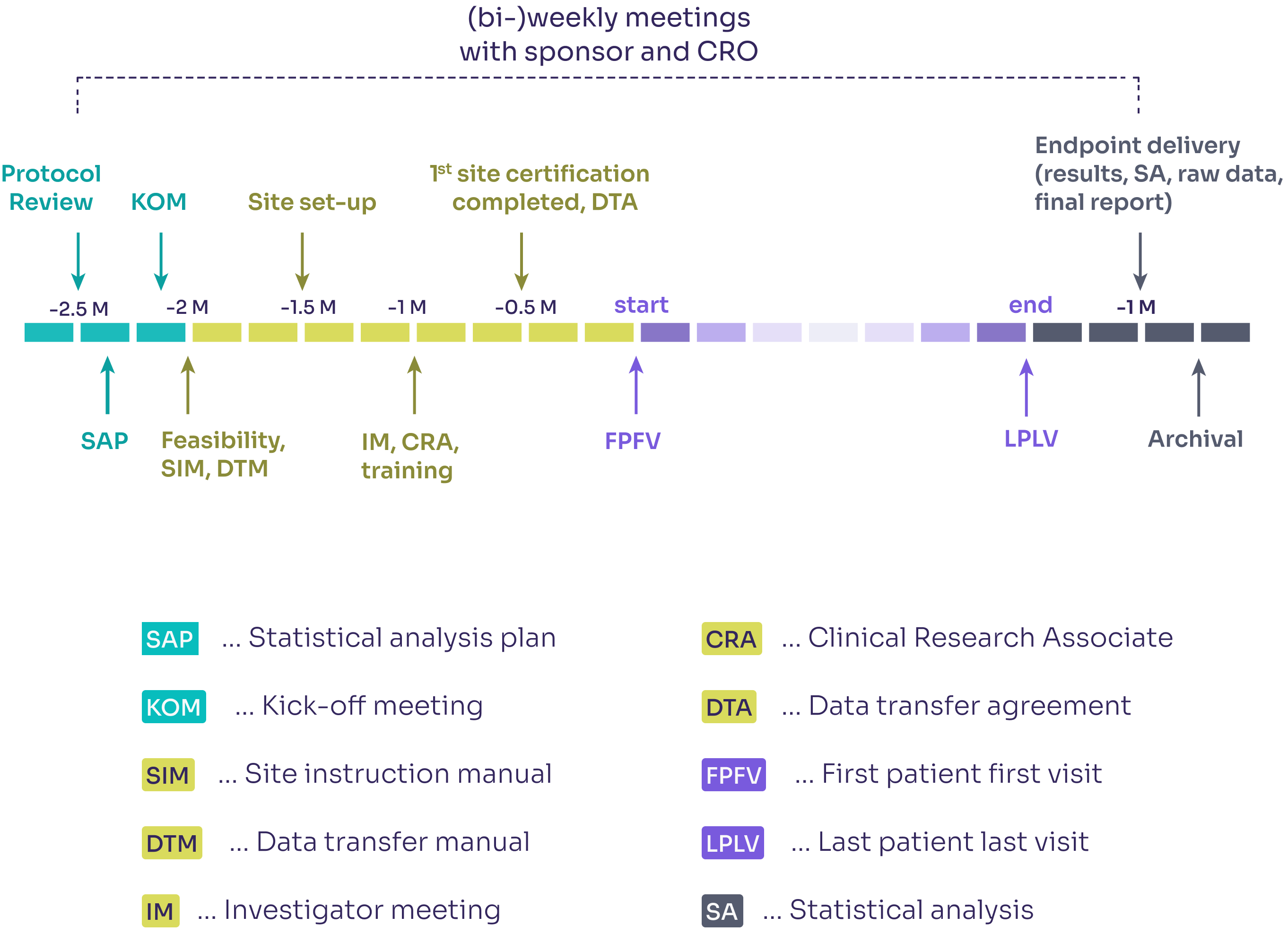
PSG study timeline blueprint
Protocol Development
With our 20+ years of CNS clinical trial experience, we partner with our clients in addressing overarching questions that strategically define the ideal trial scenario.